Update Information on Availability and Use of Treatments for Outpatients with Mild to Moderate COVID-19 Who are at Increased Risk for Severe
Early outpatient treatment of COVID-19 can avert serious, potentially life-threatening illness and reduce burden on the healthcare system.
This article is 3 years old. It was published on May 17, 2022.
Missouri healthcare providers and public health practitioners: Please contact the Missouri Department of Health and Senior Services’ Bureau of Communicable Disease Control and Prevention at 573-751-6113 or 800-392-0272 (24/7) with questions regarding this Health Advisory
Summary
The Centers for Disease Control and Prevention (CDC) is issuing this Health Alert Network (HAN) Health Advisory to update healthcare providers, public health departments, and the public about the availability and use of recommended therapies for COVID-19 and to advise against using unproven treatments that have known or potential harms for outpatients with mild to moderate COVID-19. For patients with mild to moderate COVID-19 who are not hospitalized and who are at increased risk for severe COVID-19 outcomes, several treatment options, including antiviral medications and monoclonal antibodies, are now widely available and accessible.
Systemic corticosteroids are not recommended to treat patients with mild to moderate COVID-19 who do not require supplemental oxygen; patients who are receiving dexamethasone or another corticosteroid for other indications should continue therapy for their underlying conditions as directed by their healthcare providers. Antibacterial therapy is not recommended for the treatment of COVID-19 in the absence of another indication.
Staying up to date with COVID-19 vaccination is still the best way to prevent serious outcomes of COVID19, including severe disease, hospitalization, and death.
Background
Early outpatient treatment of COVID-19 can avert serious, potentially life-threatening illness and reduce burden on the healthcare system. CDC issued a HAN Health Advisory on December 31, 2021 to address using therapeutics in the outpatient setting for people with COVID-19. At that time, Omicron cases were increasing rapidly in the United States and some COVID-19 therapeutics were in short supply. Now antivirals for COVID-19 are widely available and can be accessed with a provider prescription at pharmacies nationwide and at Test to Treat locations.
The National Institutes of Health (NIH) provides COVID-19 Treatment Guidelines. The guidelines panel provides treatment options and recommends against using systemic corticosteroids to treat patients with mild to moderate COVID-19 who do not require supplemental oxygen (Figure). Patients who are receiving dexamethasone or another corticosteroid for other indications should continue therapy for their underlying conditions as directed by their healthcare providers. Systemic corticosteroids are recommended for hospitalized patients with COVID-19 who require supplemental oxygen or higher-level respiratory support.
The guidelines panel also recommends against using antibacterial therapy for COVID-19 in the absence of another indication. Antibacterial drugs have no benefit in treating viral infections and can cause harm.
Figure. Therapeutic Management of Nonhospitalized Adults with COVID-19 (from NIH COVID-19 Treatment Guidelines, last updated: April 8, 2022)
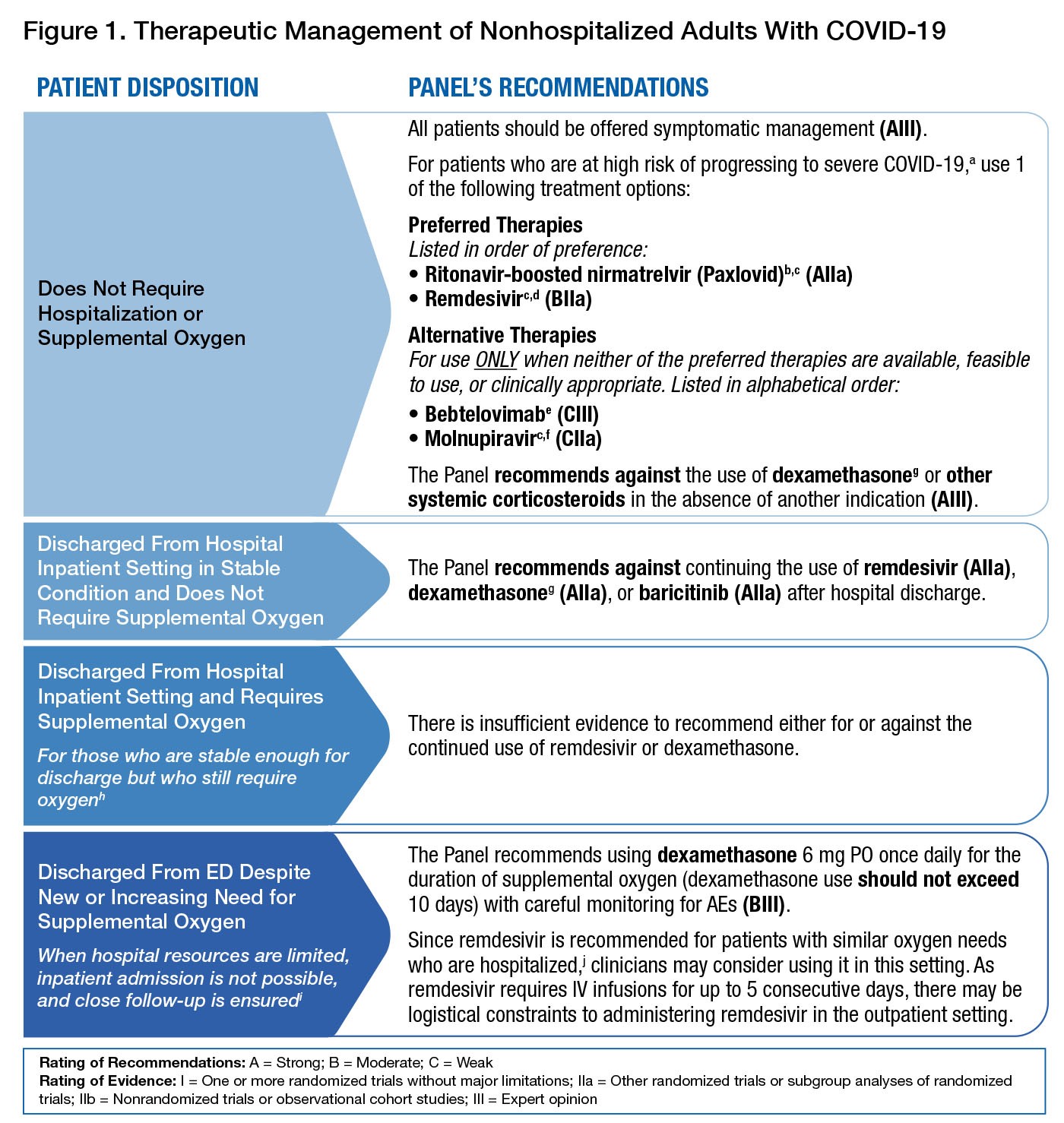
a. For a list of risk factors, see the CDC webpage Underlying Medical Conditions Associated With Higher Risk for Severe COVID-19.
b. Ritonavir-boosted nirmatrelvir has significant drug-drug interactions. Clinicians should carefully review a patient’s concomitant medications and evaluate potential drug-drug interactions.
c. If a patient requires hospitalization after starting treatment, the full treatment course can be completed at the healthcare provider’s discretion.
d. Administration of remdesivir requires 3 consecutive days of IV infusion.
e. Bebtelovimab is active in vitro against all circulating Omicron subvariants, but there are no clinical efficacy data from placebo-controlled trials that evaluated the use of bebtelovimab in patients who are at high risk of progressing to severe COVID-19. Therefore, bebtelovimab should be used only when the preferred treatment options are not available, feasible to use, or clinically appropriate.
f. Molnupiravir has lower efficacy than the preferred treatment options. Therefore, it should be used only when the preferred options are not available, feasible to use, or clinically appropriate.
g. There is currently a lack of safety and efficacy data on the use of this agent in outpatients with COVID19; using systemic glucocorticoids in this setting may cause harm.
Recommendations for Healthcare Providers
- Obtain updated information on appropriate use of clinically indicated therapeutics through NIH’s COVID-19 Treatment Guidelines.
- Prescribe COVID-19 therapeutics for patients when clinically indicated.
- There are considerable differences in efficacy, risk profiles, and use restrictions between the two oral antivirals. Healthcare providers need to be familiar with these distinctions to make clinical decisions and inform patients. In addition, initiating treatment with these oral antivirals must begin within five days of symptom onset to maintain product efficacy.
- Please see NIH’s COVID-19 Treatment Guidelines for important therapeutic considerations, such as the potential for significant drug-drug interactions with ritonavirboosted nirmatrelvir (Paxlovid) and dosing regimens for patients with renal impairment.
- Obtain information on access to outpatient COVID-19 treatments, including pharmacies where antivirals for COVID-19 are distributed and Test to Treat locations.
- Do not use dexamethasone and other systemic corticosteroids to treat patients with mild to moderate COVID-19 who do not require hospitalization or supplemental oxygen; these drugs have no proven benefit in these patients and can cause harm.
- Do not use antibacterial therapy to treat COVID-19 in the absence of another indication; these drugs have no benefit for treating viral infections and can cause harm.
- To prevent serious outcomes of COVID-19, including severe disease, hospitalization, and death, encourage all patients to remain up to date with COVID-19 vaccination.
- People who are immunocompromised or severely allergic to COVID-19 vaccines may receive tixagevimab co-packaged with cilgavimab (Evusheld), a long-acting combination monoclonal antibody therapy given by intramuscular injection for preexposure prophylaxis of COVID-19. To find Evusheld distribution locations, providers can go to the COVID-19 Therapeutics Locator, call the support line at 1-800-232-0233 (TTY 888-720-7489), or contact their individual state or territorial health planners.
Recommendations for Public Health Departments and Public Health Jurisdictions
- Maintain awareness of locations of available therapeutics within your jurisdictions, including pharmacies where antivirals for COVID-19 are distributed and Test to Treat locations.
- Communicate ongoing and up-to-date information on therapeutics for COVID-19 and their availability to healthcare providers within your jurisdiction. 3. Disseminate information for the Test to Treat call center at 1-800-232-0233 (TTY 1-888-720- 7489) which provides information in more than 150 languages, and for the Disability Information and Access Line at 1-888-677-1199.
Recommendations for the Public
-
If you test positive and are an older adult or someone who is at increased risk of getting very sick from COVID-19, treatment is available. Contact a healthcare provider right away after a positive test to determine if you are eligible for treatment, even if your symptoms are mild. You can also visit a Test to Treat location and, if eligible, receive a prescription from a provider at that location.
-
Follow CDC guidance on testing for COVID-19 and use the Test to Treat locator or call 1-800- 232-0233 (TTY 1-888-720-7489) to find a testing location that can provide treatment if you test positive.
- Don’t delay: Treatment must be started within the first few days of when your symptoms started to be effective.
- Staying up to date with COVID-19 vaccination is still the best way to prevent serious outcomes of COVID-19, including severe disease, hospitalization, and death.
For More Information
- CDC COVID-19 Treatment website
- NIH COVID-19 Treatment Guidelines
- NIH COVID-19 Treatment Guidelines: Therapeutic Management of Nonhospitalized Adults with COVID-19
- Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC
- NIH COVID-19 Treatment Guidelines: Prevention of SARS-CoV-2 Infection
- Office of the Assistant Secretary for Preparedness & Response (ASPR) Test to Treat website
- U.S. Food and Drug Administration COVID-19 Therapeutic Product Emergency Use Authorizations
- CDC COVID Data Tracker
References
- Geller AI, Lovegrove MC, Lind JN, Datta SD, Budnitz DS. Assessment of outpatient dispensing of products proposed for treatment of prevention of COVID-19 by U.S. retail pharmacies during the pandemic. JAMA Intern Med 2021;181:869-72.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2776456 - Tsay SV, Bartoces M, Goulin K, Kabbani S, Hicks, LA. Antibiotic prescriptions associated with COVID-19 visits among Medicare beneficiaries, April 2020 to April 2021. JAMA 2022. https://jamanetwork.com/journals/jama/fullarticle/2791077
- Bradley MC, Perez-Vilar S. Chillarige Y, Dong D. Martinez AI, Weckstein AR, Dal Pan GJ. Systemic corticosteroid use for COVID-19 in U.S. outpatient settings from April 2020 to August 2021. JAMA 2022. https://jamanetwork.com/journals/jama/fullarticle/2791078
- Yao TC, Huang, YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events. Ann Intern Med 2020;173:325-30. https://www.acpjournals.org/doi/10.7326/M20-0432
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693-704. https://www.nejm.org/doi/full/10.1056/nejmoa2021436
- Crothers K, DeFaccio R, Tate J, et al. Dexamethasone in hospitalised coronavirus-19 patients not on intensive respiratory support. Eur Resp J 2021. https://erj.ersjournals.com/content/early/2021/11/18/13993003.02532-2021
- Li Q, Li W, Jin Y, et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect Dis Ther 2020;9:823-36. https://pubmed.ncbi.nlm.nih.gov/32880102/
-
Department:
Department of Health
-
Topic:
Most Read News
- Mayor Cara Spencer Takes Office as the 48th Mayor of St. Louis She emphasized that better days are ahead for St. Louis and pledges to work collaboratively with all stakeholders
- CDA Announces more than $16 Million Available in 2025 Neighborhood Transformation Grants Funding includes CDBG, HOME, HOME-ARP, PRO Housing, and Economic Development Sales Tax investments to support affordable housing production, neighborhood plan implementation, and community green activation of vacant lots.
- New Data Show St. Louis City Continues To See Decrease in Homicides and Other Crime Newly released crime data for August 2024 shows that the City of St. Louis continues to successfully decrease the amount of crime, including homicides, occurring in the City.